How to Handle Medical Waste in Med Spas and Non-Healthcare Settings

Medical waste removal is not solely within the remit of the healthcare industry. Any business that generates biohazardous medical waste must adhere to the proper medical waste disposal regulations and guidelines to protect its employees, customers, and communities. Medical spas – commonly known as medspas – and other medicine-adjacent services generate biohazardous waste, and it’s incumbent upon the management of these facilities to understand how to safely and effectively meet their medical waste removal challenges.
IN THIS BLOG:
1 / Introduction
2 / Medspas
5 / The advantages of expert support
Introduction
Healthcare settings aren’t the sole generators of regulated medical waste (RMW). Businesses that provide body modification services involving invasive or minimally invasive procedures handle biohazardous medical waste on a routine basis. This waste might include sharps (needles and sharp instruments that pierce the skin), blood-soaked gauze, protective covers, swabs, and personal protective equipment like gloves.
While medspas offer healthcare-adjacent services, they do not specifically treat infectious illnesses, so it is easy for workers in such environments to become less-than-attentive about maintaining safe disposal practices. For these kinds of businesses, routine training in safe medical waste removal and handling is critical. Failure to adhere to proper medical waste removal and handling protocols can put the health and safety of employees, clients, and communities in jeopardy.
Medspas
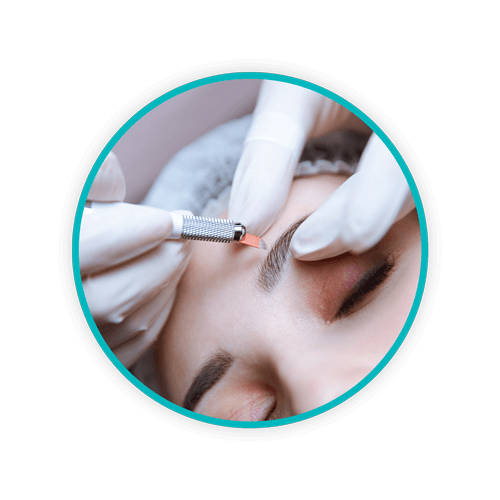
Medspas – facilities that offer the combined services of aesthetic medical clinics and day spas – produce a variety of medical waste products. Not only do medspas discard sharps, there are also pharmaceutical waste products like dermal fillers, botulinum toxins, and anesthetics. Additionally, some facilities offer platelet-rich plasma therapy – injections of a patient’s own blood – to achieve particular cosmetic outcomes. All these services generate significant biohazardous waste that must be segregated and handled carefully. Improper medical waste removal – particularly of sharps – puts workers, clients, and people in the community at risk of exposure to bloodborne pathogens that have the potential to cause serious illness and even death. These include:
- Hepatitis B (HBV)
- Hepatitis C (HCV)
-
Human Immunodeficiency Virus (HIV)
The ingredients for some pharmaceuticals used for many medspa treatments can be considered acute hazardous waste, and must be handled and disposed of according to specific regulatory guidelines. The Federal Environmental Protection Agency has issued guidance about the handling of hazardous waste pharmaceuticals, and individual states have their own regulations and standards for disposal. There are also standards issued by the Centers for Disease Control and Prevention and the Occupational Safety and Health Administration. It is the responsibility of the medspa facility to learn and adhere to the guidelines, which can be complex.
Research laboratories
Research laboratories might handle a broad assortment of specimens, including materials from humans, plant species, and animals. The composition and volume of the waste streams generated by research labs will largely depend upon the function of the individual laboratories. Every lab has a particular type of characteristic waste, but any waste that could be classified as biohazardous, pathological, or chemical must be handled and disposed of in a manner that adheres to federal and state regulations.
Plans for appropriate waste segregation, storage, and disposal of laboratory waste are exceptionally urgent for facilities that routinely handle biohazardous waste. According to the Centers for Disease Control and Prevention, laboratory waste – whether from medical research laboratories, clinical laboratories, or bioresearch laboratories – has the potential for causing the most significant and problematic contamination if handled improperly. Microbiological waste consists of artificially amplified microbial populations, untreated cultures, both live and attenuated vaccines, and contaminated antibiotic production waste. These agents present the highest risk of large-scale transmission of infectious disease.
Chemical waste generated by research laboratories must be handled scrupulously. Chemical agents have to be evaluated individually to determine whether the volumes accumulated in waste streams are considered hazardous.
Marijuana dispensaries

Because marijuana is considered a Schedule I Controlled Substance, dispensaries have to be extremely careful with the disposal of cannabis waste products. Tetrahydrocannabinol (THC), the sometimes potent chemical in many cannabis products, can harm ecosystems and even poison wildlife if disposed of carelessly.
Different states have different guidelines for cannabis medical waste removal. In most states where marijuana has been either legalized or decriminalized, the product must be rendered unusable, though the guidance differs depending upon the state.
In California, retailers and testing laboratories that are licensed under the Bureau of Cannabis Control or the Manufactured Cannabis Safety Branch (MCSB) must render their waste unrecognizable and unusable. However, growers and processors licensed under CalCannabis do not have to render their waste unusable.
In Illinois, dispensaries are required to render their waste unusable by integrating it into other bulk waste, rendering the product less than 50 percent cannabis by volume. In Colorado, medical marijuana waste must be incorporated into substances that render it non-consumable.
How you can benefit from the expertise of Daniels Health
Having the support of an experienced medical waste removal service can help businesses that aren’t healthcare providers yet routinely generate biohazardous waste navigate the complex processes of medical waste removal. Daniels Health offers products, guidance, and consultation services for diverse organizations that want to stay compliant with often highly complicated regulations. To learn more about our products and services or to request a quote, please visit our information page.
REQUEST A CONSULTATION VISIT OUR MED-SPAS PAGE
