USP <800> Standard: the Basics
Looking for tools to educate your staff or self on the USP <800> federal standard? We have put together this guide to get you started on the road to compliance regarding handling Hazardous Drugs (HDs) in a healthcare setting. Below you’ll find answers to some common questions as well as downloadable resources!
Did you know that “about 8 million U.S. healthcare workers are potentially exposed to hazardous drugs, including pharmacy and nursing personnel, physicians, operating room personnel, environmental services workers, workers in research laboratories, veterinary care workers, and shipping and receiving personnel?”1 That’s a lot of lives that could be negatively affected if the proper safety measures aren’t put into place. USP 800 was put into place to protect you because although as a healthcare worker your main priority is caring for your patients –
“Patient safety and employee safety can’t be separated – they have to go together.”
This guide will cover:
2 / What facilities should be ready to implement USP <800> most?
3 / An interview with our in-house hazardous waste expert, Adam Thompson, on USP <800>
4 / Training materials for how to implement USP <800>
5 / Recap: What resources are available
What does “USP” even stand for?
United States Pharmacopeia, of course! Try saying that three times fast. The history of how USP came to be is actually quite interesting and may help you further understand “the why” of what they do. If you’re not interested in a brief history lesson, feel empowered to skip ahead!
It began with Lodvice dal Pozzo Toshcanelli, a 15th century Italian physician. Toschanelli began recording what formulas provided effective and safe drug therapy. He then produced a small book of his findings that the local guild of pharmacists would go on to reference for quality standards. Think of it as an original “best practice” guide.
As history continued, and legislative regulatory standards for pharmaceuticals were yet to be found, pharmacists would reference “drug books.” The inaugural United States Pharmacopeia (USP), was created by a group of American Physicians in 1820. This was the next phase of the “drug book.” The USP outlined formulas for over 200 drugs “considered to be the ‘most fully established and best understood.’”2
Time passed and eventually the Food and Drug act came along in 1906 – this added another level of authority to USP. Now, with public involvement and clear-intentions, scientific experts volunteer to create and establish these new USP standards. For deeper look at the origins of USP’s history, view their site here.
See, history can be pretty cool! This shows that safe drug handling, creation, and distribution has been important to healers for centuries. As our knowledge evolves, so do our standards of safety – and this is where USP800 comes in.
First off, what facilities should care about the USP <800> standard?
Well, any that are handling NIOSH Hazardous Drugs which can range from antineoplastic, non-antineoplastic, and non-antineoplastic drugs that have adverse side effects.
Basically USP <800> is going to be a big deal for all facilities but especially:
What are antineoplastic drugs?
So glad you asked. According the CDC, “Antineoplastic drugs are medications used to treat cancer. Antineoplastic drugs are also called anticancer, chemotherapy, chemo, cytotoxic, or hazardous drugs.”3 You can view NIOSH’s full List of Antineoplastic or other Hazardous Drugs in Healthcare Settings here. You can also download a list we put together for you based on NIOSH’s information:
These types of drugs are known to cause adverse health effects – which is why USP <800> is implementing a standard for safer handling. Mishandling of Hazardous Drugs can lead to damage in major organs and cause reproductive challenges.
Now we call to the stand, an expert witness in Hazardous Waste, Adam Thompson!
Well an in-house expert with over 5 years’ experience advising on the management of hazardous waste. Adam is fantastic at putting somewhat dense concepts, like USP <800>, into easy-to-understand terms. I often email him to request a “for dummies” version of various hazardous waste questions because it’s confusing for me too! Let’s see what he has to say about USP <800>.
Why has USP <800> been put into place?
“To protect people from the dangers of drugs that can cause reproductive health issues. This standard has identified a list of ‘hazardous drugs’ which are separate from what we have previously understood as hazardous. Think of this as a brand-new list in addition to what you already know. If a medication is on this list, there are specific standards for how you have to handle it and how it has to be packaged. The standard is subtitled ‘Hazardous Drugs – handling in Healthcare Settings.’ This does not elaborate on best methods of disposal; however, Daniels Health has provided a recommendation on best disposal methods.”
Poster: Understanding USP <800>
Who does this impact in healthcare and what do they need to do?
“This impacts everyone in healthcare – anyone who may potentially handle a hazardous drug. Essentially USP <800> sets the framework for creating Standard Operating Procedures for each facility for how they need to handle these drugs. The main takeaway is that your facility should develop a safe plan and train their staff on how to handle and package USP <800> drugs.”
Where can people read more about USP <800>?
“Here. For example, on page 4 you can read about Facilities and Engineering Controls. In this section you learn that hazardous drugs under this standard:
- Can’t be stored on the floor or in areas prone to specific types of natural disasters. Kind of a no brainer but still important!
- If stored on a shelf, the shelves must have raised lips so it can’t fall or be knocked off.
- You cannot store sterile and non-sterile hazardous drugs together.
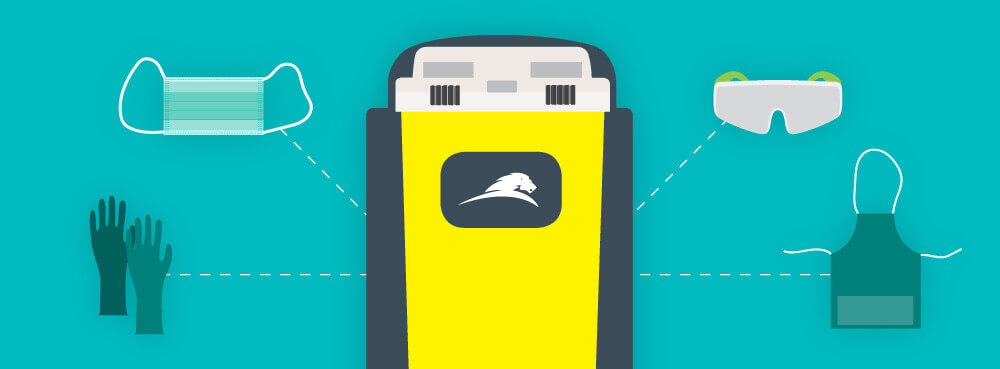
For handling of these hazardous drugs, you have to wear PPE including double gloves. Two pairs of chemotherapy gloves are required for administering antineoplastic Hazardous Drugs (HDs). Reference your site’s occupational safety plan. It is the responsibility of your facility to train you and your colleagues on the appropriate PPE to wear and safe handling methods.
Appropriate PPE must be worn when handled HDs including during:
- Receipt
- Storage
- Transport
- Compounding (sterile and nonsterile)
- Administration
- Deactivation/decontamination, cleaning, and disinfecting
- Spill Control
- Waste Disposal
In section 11.1 Labeling, you can learn that all HDs must have a special label on them that say they’re hazardous drugs. This is very clear.
Following right after that, section 11.4 Disposal states, ‘Disposal of all HD waste, including, but not limited to, unused HDs and trace-contaminated PPE and other materials, must comply with all applicable federal, state, and local regulations.’ So while USP <800> is not enforced by RCRA, it is the new standard of safety that all sites need to adopt to protect their staff.”
Brief interruption from me, Megan (the person writing this), jumping back in to let you (the reader) know that Daniels Health has an entire guide on USP 800 and PPE. Our five-page instructional guide outlines PPE and Engineering controls for working with NIOSH Hazardous Drugs in healthcare settings.
Okay, now that you know about that – back to Adam!
What other trainings may be beneficial for staff?
“Global Harmonized System training could be helpful. I know Daniels offers this training with their Compliance Program. This ensure staff can quickly read and understand hazard pictograms and the labeling of chemicals. Staff must also have an SDS for each hazardous chemical they use – a SDS database is also available in Daniels’ Compliance Program.”

Daniels is recommending the Chemosmart container as a cost-effective and compliant solution for USP <800> items and PPE, why?
“Many of the items on the HD list are chemotherapy drugs. Anything that is trace has less than 3% volume of original material and is no longer regulated by RCRA. So that’s why those can go in Chemosmart. Again, this is the best recommended practice by Daniels but ultimately the site’s decision. The PPE used could also have trace amounts on it. It is simpler to discard all PPE as trace. Disposing of items in the Chemosmart would be more cost-effective than over-classifying the items and putting them in a black bin.. ”
Poster: Understanding USP <800>
And that concludes Adam’s interview! Thanks, Adam!
Now that you’re armed with some USP <800> base knowledge and advice, let’s recap all the resources available to you:
Featured USP <800> Resources:
- Understanding the USP 800 Standard
- NIOSH Antineoplastic Hazardous Drug List
- USP <800> Personal Protective Equipment
- Daniels USP <800> System
Did you find this guide helpful for understanding the USP <800> standard? If so, share it! To learn more about how Daniels Health’s Chemosmart system provides hands-free USP 800 disposal or more about our GHS hazcom training offering contact us!
We also have an entire webpage dedicated to USP <800> with all the resources and our recommended solutions.
Resources
¹ Centers for Disease Control and Prevention Hazardous Drug Exposures in Healthcare. 2018
2 Global Health. “What is Pharmacopeia?”. USP. 2014
3Centers for Disease Control and Prevention Reproductive Health and the Workplace. 2019
Let's Talk!
Your time is valuable, and we don’t want to play hard to get. You can either phone us directly on the details listed on our contact page, or feel free to fill out this short form and one of our team members will get back to you as quickly as possible.
