
USP800 Personal Protective Equipment
Aligning with USP800, this resource pictorially illustrates when PPE and safety precautions are required for different drug categories.
On December 1st 2019, a new Standard governing the handling of hazardous drugs such as warfarin, and fluconazole trace residue will be enforced. Under the new NIOSH governed Standard, gloves, masks, foot covers, bunny suits and gowns that are used in the administering of any USP <800> classified substance are now defined ‘contaminated’, and therefore fall into a new disposal and waste containment. We help you navigate what this looks like for your facility.
| Product Code: | c65b9d6666f4 |
|---|---|
| Media type: | Flyer |

Aligning with USP800, this resource pictorially illustrates when PPE and safety precautions are required for different drug categories.

How to utilize Daniels' USP800 waste containment system for isolation units and general wards post-procedure.

This simple education poster outlines what is classified as non-sharp Trace hazardous medication waste (USP800).
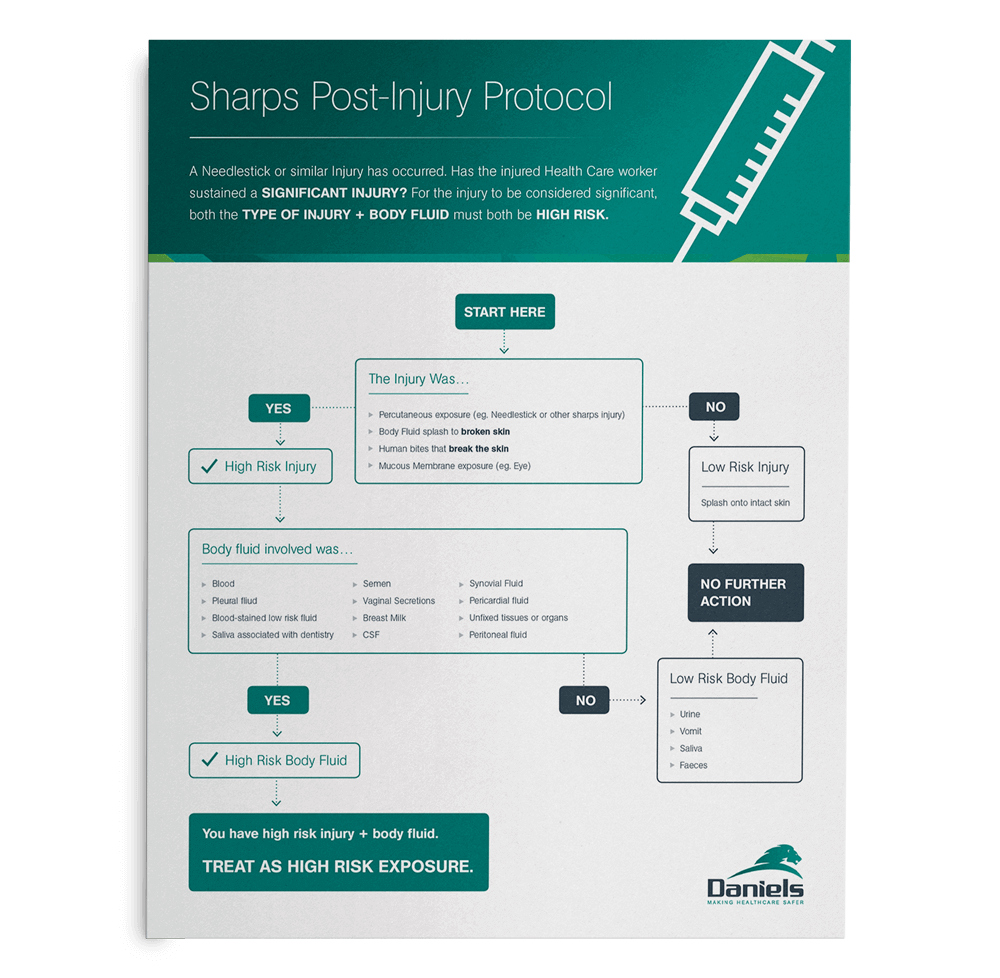
Guide on how to determine the severity of a sharps injury and what to do if a sharps injury occurs.
Your time is valuable, and we don’t want to play hard to get. You can either phone us directly on the details listed on our contact page, or feel free to fill out this short form and one of our team members will get back to you as quickly as possible.
Notifications